Research: Microbial Biosynthesis
Our goal is to establish principles for the high-volume production of plant food components, such as amino acids, vitamins, lipids, and other valuable natural products, in industry-relevant microbes.
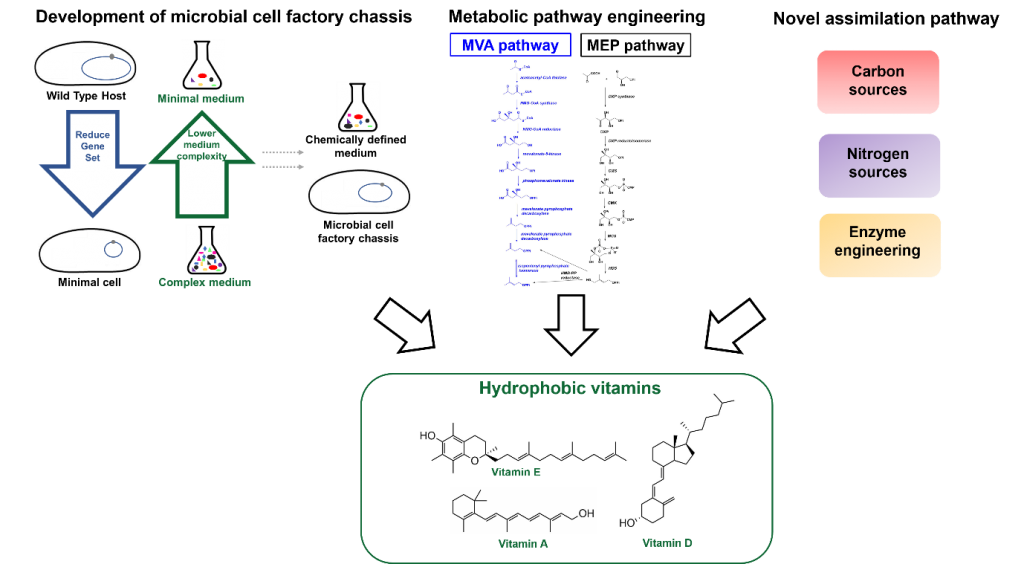
- We are developing microbial biosynthetic processes that can efficiently produce hydrophobic vitamins from sugars
- We are systematically editing genome of industrial microbial strains to produce valuable chassis strains
Metabolic Engineering of Microbes to Produce Phytochemicals
As a fast-growing component of the agricultural sector, the phytochemicals industry is facing a surging global demand, which aggravates the need for improved agricultural productivity. Despite advances in agricultural precision, the low abundance of phytochemicals in conventional agriculture makes it appealing to transition the production of these phytochemicals to engineered microbial platforms. We aim to develop novel technologies and principles for effective metabolic engineering of microbes to achieve high volume production of various phytochemicals.
The SINSKEY Lab and the STEPHANOPOULOS Lab at MIT and the ZHOU lab at NUS have focused on engineering Corynebacterium glutamicum to produce high value, hydrophobic plant food compounds, such as lycopene (Vitamin A), Vitamin E, and squalene. Many of these compounds are currently produced through the use of genetically modified (GM) crops to improve yields, but overall production still remains low due to inconsistent grain yields, GM crop regulations, and anti-GM crop activism. Chemical synthesis of these compounds is possible, but these procedures are costly, detrimental to the environment, and yield low amounts. In order to produce these compounds in a sustainable and cost-effective manner, we are engineering new pathways into C. glutamicum using synthetic biological circuits to reduce starting cost and improve yields. Many of these pathways originate from plant biosynthetic pathways and integrated into the C. glutamicum genome. In order to improve the ability to reprogram industry-relevant workhorse strains such as C. glutamicum for heterologous metabolite production, we are developing new technologies to reduce the overall genome of this organism that will provide a number of benefits. This organism is expected to be superior compared to the wild type in terms of genomic stability (no mobile genetic elements), predictability (less reactions to model, reduced number of futile cycles, etc.), and productivity (less carbon/energy wasted on by-products). In addition, due to an absence of side-reactions and knowledge gained during the development of the minimal genome chassis strain, subsequent strain development for almost any kind of product should be faster.